Abstract
Introduction: UCART22 is a genetically modified allogeneic T-cell product manufactured from non-HLA matched healthy donor cells. Donor-derived T-cells are transduced using a lentiviral vector to express the anti-CD22 chimeric antigen receptor (CAR; anti-CD22 scFv-41BB-CD3ζ) and are further modified using Cellectis' TALEN ® technology to disrupt the T-cell receptor alpha constant (TRAC) and CD52 genes to minimize risk of graft-vs-host disease (GvHD) and allow use of anti-CD52-directed drugs for lymphodepletion (LD).
Preliminary results from the phase 1, open-label, dose-escalation BALLI-01 study (NCT04150497) in patients (pts) with R/R B-ALL showed that UCART22 is tolerable and shows anti-leukemic activity after LD with fludarabine and cyclophosphamide (FC) (Jain, ASH 2020). Host T-cell recovery was observed in all pts between days (d) 7-28. Subsequent UCART22 dose-finding cohorts utilized a new LD regimen that included the addition of alemtuzumab to FC ("FCA"), which could potentially deepen and sustain host T-cell depletion and promote CAR T-cell expansion and persistence.
Methods: Eligibility criteria include age 15‒70 yrs, adequate organ function, ECOG PS ≤ 1, B-ALL blast CD22 expression ≥ 70% by flow cytometry. Pts must have received ≥ 1 prior standard chemotherapy regimen and 1 salvage regimen. After LD with FCA (F 30 mg/m 2 × 3d, C 0.5 g/m 2 × 3d, A 20 mg/d × 3d), pts receive a single infusion of UCART22 at ~1 ×10 6 cells/kg (FCA-DL2) or ~2.5 ×10 6 cells/kg (FCA-DL2i). The primary endpoint is the safety, tolerability, and MTD of UCART22. DLTs are assessed over a 28d observation period after UCART22 infusion. Additional endpoints include anti-leukemic activity per investigator assessment (NCCN criteria), and the expansion, trafficking, and persistence of UCART22 (assessed in peripheral blood [PB] and bone marrow [BM] by phenotypic analysis using flow cytometry and vector copy number [VCN] using qPCR). Immune reconstitution is assessed by flow cytometry.
Results: As of 01 July 2021, a total of 13 pts had provided consent: 3 failed screening, 1 discontinued after LD, and 9 pts received UCART22. Enrollment in FC-DL1 (n = 3), FC-DL2 (n = 2), and FCA-DL2 (n = 3) was complete, and 1 pt had been enrolled in FCA-DL2i.
All 3 pts in FCA-DL2 (median age 29 yrs [range 29‒51]; 1 male) had discontinued at data cutoff and 1 remained in long-term follow-up. Pts had received a median of 5 (4-6) prior Tx, including blinatumomab for all 3 pts, inotuzumab for 2 pts, autologous CAR19 Tx for 1 pt. Median BM blast percentage prior to LD was 92% (88-98).
One pt in FCA-DL2 had Tx-related TEAEs of G1 CRS (requiring a single administration of tocilizumab) and G2 pruritus; both events resolved. Two pts had serious TEAEs not related to UCART22 or FCA: 1 pt had G3 hyperbilirubinemia and febrile neutropenia and 1 pt had G1 pyrexia. No pts had GvHD, ICANS, or protocol-defined DLTs. Two pts experienced 4 infectious TEAEs (BK virus [G1 n = 1], candida infection [G2 n = 2], CMV infection [G1 n = 1]). No G ≥3 infections were reported.
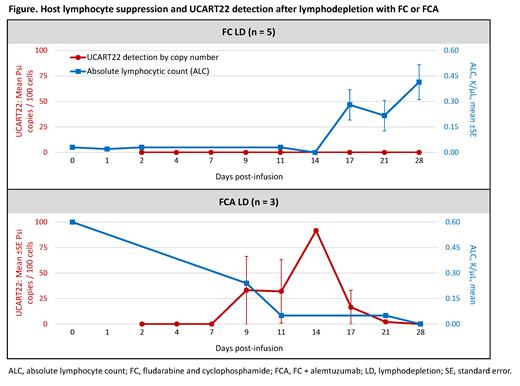
One pt had a blast reduction consistent with CRi (from 97% to 0.4%; ANC 1.07 ×10 9/L), and 1 pt had a significant blast reduction from 92% to 27%. Host lymphocytes remained suppressed throughout the 28d DLT observation period for all 3 pts in the FCA-DL2 cohort, correlating with UCART22 proliferation and > 2-fold increases in inflammatory cytokines (including IL-6, IL-10, IFN-γ, and CRP) in 2 pts (Figure). In 1 pt, peak UCART22 was detected in PB by flow cytometry on d9 (11 cells/µL) and by VCN on d14 (91 copies/100 cells), and in BM on d14 (5 cells/µL; 56 copies/100 cells); in the second pt, UCART22 was detected in PB by flow cytometry and VCN on d11 (5 cells/ µL; 0.83 copies/100 cells).
Conclusions: The FCA LD regimen was well tolerated and associated with extended host lymphocyte suppression and UCART22 expansion. No Tx-related serious TEAEs were reported at FCA-DL2, and 2 pts achieved significant blast reductions. Detection of UCART22 occurred as early as d9 after infusion and was associated with increases in inflammatory cytokines. Overall, these data support the safety and activity of UCART22 after FCA LD in pts with R/R B-ALL. Enrollment in the FCA-DL2i cohort is ongoing and updated data will be presented at the meeting.
Jain: Precision Biosciences: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Incyte: Research Funding; Pharmacyclics: Research Funding; Janssen: Honoraria; ADC Therapeutics: Honoraria, Research Funding; TG Therapeutics: Honoraria; Fate Therapeutics: Research Funding; AstraZeneca: Honoraria, Research Funding; Beigene: Honoraria; Aprea Therapeutics: Research Funding; AbbVie: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Servier: Honoraria, Research Funding. Roboz: Glaxo SmithKline: Consultancy; Jasper Therapeutics: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; Helsinn: Consultancy; Daiichi Sankyo: Consultancy; Bayer: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Blueprint Medicines: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Astex: Consultancy; Agios: Consultancy; Otsuka: Consultancy; Actinium: Consultancy; MEI Pharma - IDMC Chair: Consultancy; AbbVie: Consultancy; Jazz: Consultancy; Janssen: Research Funding; Celgene: Consultancy; Mesoblast: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy. Konopleva: Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Forty Seven: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Cellectis: Other: grant support; KisoJi: Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Stemline Therapeutics: Research Funding; AstraZeneca: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding. Liu: Agios; NGM Biopharmaceuticals; BeiGene: Consultancy; BMS; Karyopharm; Miltenyi: Research Funding; SITC: Honoraria. Schiller: Elevate: Research Funding; Karyopharm: Research Funding; Genentech-Roche: Research Funding; PrECOG: Research Funding; Samus: Research Funding; Arog: Research Funding; Abbvie: Research Funding; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; Regimmune: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Mateon: Research Funding; Actuate: Research Funding; FujiFilm: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gamida Cell Ltd.: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Trovagene: Research Funding; Celator: Research Funding; Deciphera: Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Sangamo: Research Funding; Takeda: Research Funding; Onconova: Research Funding; Geron: Research Funding; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Tolero: Research Funding; Constellation Pharmaceuticals: Research Funding; Forma: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; Bio: Research Funding; Ono-UK: Consultancy, Research Funding; Delta-Fly: Research Funding; Daiichi-Sankyo: Research Funding; Astellas: Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding, Speakers Bureau; Pharma: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Biomed Valley Discoveries: Research Funding; Eli Lilly: Research Funding; ASH foundation: Other: Chair-unpaid; Sellas: Research Funding; Ono: Consultancy; Incyte: Consultancy; Ariad: Research Funding; AstraZeneca: Consultancy; Kaiser Permanente: Consultancy; Cyclacel: Research Funding; MedImmune: Research Funding; Ambit: Research Funding; Leukemia & Lymphoma Society: Research Funding; Bluebird Bio: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Whitfield: Cellectis: Current Employment. Haider: Cellectis: Current Employment, Current equity holder in publicly-traded company. Zernovak: Cellectis: Current Employment, Current equity holder in publicly-traded company; Janssen: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Frattini: Cellectis: Current Employment, Current equity holder in publicly-traded company; Bristol Myers Squibb: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Brownstein: Cellectis: Current Employment, Current equity holder in publicly-traded company; Bristol Myers Squibb: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Larson: Gilead: Research Funding; Epizyme: Consultancy; Astellas: Consultancy, Research Funding; Cellectis: Research Funding; Rafael Pharmaceuticals: Research Funding; CVS/Caremark: Consultancy; Takeda: Research Funding; Novartis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal